PGHD in the HACLab

- Log in to post comments
Background
While most healthcare data comes from insurance claims and EHR “exhausts”, these sources have clear limitations. Patient-generated health data (PGHD) are health-related data created, recorded, or gathered by or from patients (or family members or other caregivers) to help address a health concern. HACLab researchers leverage PGHD – i.e. patient-reported outcomes (PROs) on symptoms collected via text message and biometric data collected via wearable technology – to run trials to investigate how patient-generated data may improve clinical decision-making. In addition, we are on the forefront of advancing digital health applications more broadly, specifically in oncology. We also investigate how this data may be used at scale to predict future outcomes and understand trajectories of functional and clinical outcomes. This blog highlights this unique research area by reviewing a case study of one of our clinical trials: PROStep: A Feasibility Trial Using PROs and Step Data to Monitor Patients With Lung and GI Cancers.
PROStep Case Study
Study Protocol
https://bmjopen.bmj.com/content/12/5/e054675
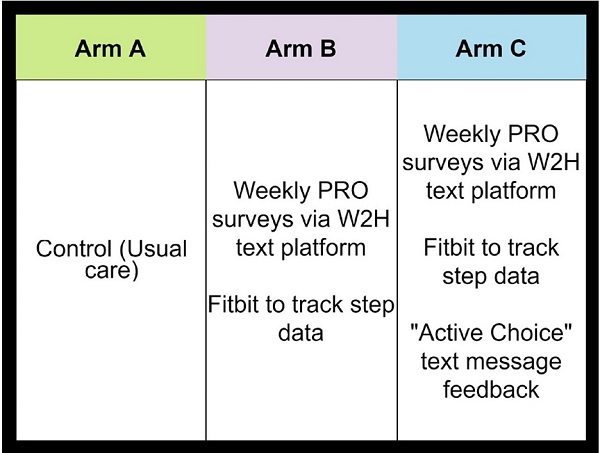
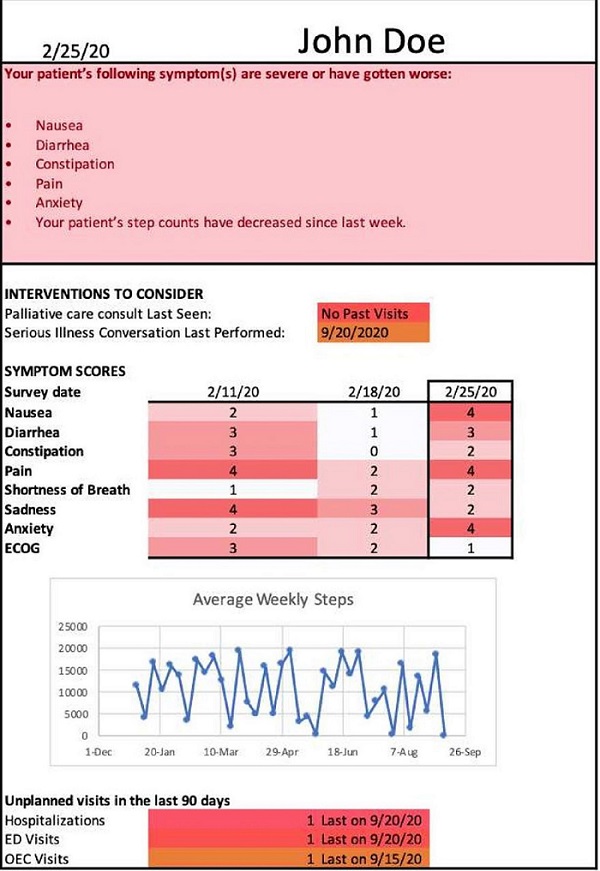
PROStep was a single-center prospective RCT where patients with metastatic gastrointestinal or lung cancers were randomized into one of three arms. HACLab researchers enrolled patients with stage IV GI and lung cancers undergoing chemotherapy. Over 6 months, patients in both intervention arms received PROStep—weekly text message–based symptom surveys and passive activity monitoring using a wearable accelerometer. PGHD were summarized in dashboards given to patients' oncology team before appointments. We also utilized behavioral economic principles – specifically active choice – by integrating an additional text-based active choice prompt to discuss worsening symptoms or functional status with their clinician at their upcoming visit. Ultimately, our objective with PROStep was to answer the following: What is the impact of integrating remote collection of PGHD with patient nudges on symptom and functional status understanding for patients with advanced cancer and their oncology team?
Results
https://ascopubs.org/doi/10.1200/OP.23.00048
PROStep ran from November 19, 2020, to December 17, 2021 enrolling a total of 108 patients (55% male, 81% White, and 77% had GI cancers). Patient-reported clinician understanding did not differ between control and intervention arms for symptoms or functional status. Patients receiving PROStep reported high understanding of symptoms and functional status from their oncology team, although this did not differ from controls.
Takeaways:
- Patient-reported clinician understanding did not differ between control and intervention arms for symptoms
- Patient-reported clinician understanding did not differ between control and intervention arms for functional status
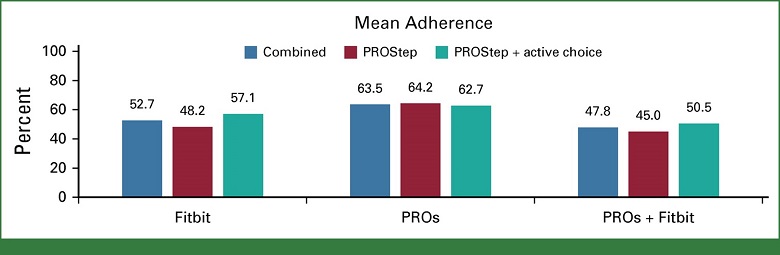
- Among intervention patients, combined patient adherence to weekly symptom reports and daily activity monitoring was 64% (Arm B) and 53% (Arm C)
- Intervention patients reported low burden from wearing the accelerometer and completing surveys
Next steps w/ PROStep
Future work should aim to improve patient adherence to remote patient-reported outcome and activity data collection and create actionable data for oncology clinicians to improve symptom management and discussions about treatment and goals of care.
We have conducted a separate analysis (pending publication) analyzing the association between wearable step counts and patient-reported outcomes, in an attempted to define objective step count thresholds that correspond to different performance status metrics. We also have assessed associations between step count thresholds and future symptom decline, to determine whether declines in step count can be used to predict future symptom burden.
Future work (more to come!) will also analyze how step counts dynamically change during chemotherapy cycles and, as part of a multi-national consortium, attempt to define an “objective performance status” that could be used in clinical trial assessment.
HACLab and PGHD moving forward
Based on our experience using wearable accelerometers, we are pursuing a novel project collecting wearable step count data in order to identify subcohorts of older adults who may benefit from proactive dose reductions in chemotherapy. The study, if successful, will be the first of its kind to use “digital geriatric assessments” to tailor chemotherapy dosing for older adults. We anticipate enrollment to start in late summer/early fall 2024 at three Penn Medicine sites: Penn Medicine Princeton Health, Lancaster General Hospital, and Pennsylvania Hospital.
